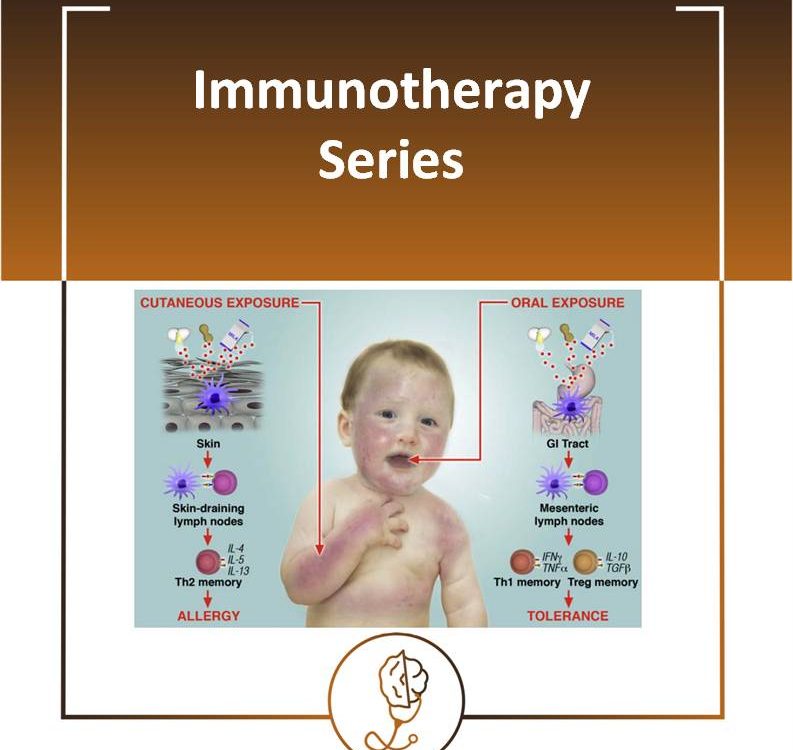
Peanut Allergy

Hayfever / Allergic Rhinitis
04/08/2020
Pollen Food Syndrome
16/08/2020The incidence of peanut allergy has been increasing significantly in developed countries.
Many reasons have been found for such growth, but so far no proper treatment has been found to deal with it.
Often, antihistamines are the first choice when there is an allergic reaction, with the occasional need to use adrenaline as well.
Most of the symptoms start in childhood and persist throughout life.
Due to this, the search for ways to deal with this specific allergy is ongoing, though not matching current needs.
As such, today, I will focus on a promising medication that might lead to a significant change in the way we deal with peanut, and possibly, tree nut allergy.
In a Phase 2a randomized placebo control trial, running for six weeks, a single injection of Etokimab showed that it could desensitize peanut allergic adults.
- Food challenges, skin prick tests and blood tests were done on days 15 and 45, after the injection
- 73% (first challenge) and 57% (second challenge) passed a food challenge with 275mg of peanut protein (versus 0% in the control group)
- Several immune mediators were also reduced
They concluded that it could potentially desensitize peanut-allergic participants and possibly reduce atopy-related adverse events.
I understand the first part, but what is the connection to atopy?
Let me explain.
- IL33 belongs to a group of proteins (cytokines) important in cell signalling (basically part of the immune cascade).
- It also belongs to a small group called alarmins, which are biomolecules that can initiate and maintain a non-infectious inflammatory response (like in allergies).
- It is also known to be released or elevated when there is skin damage, like in atopic dermatitis or eczema (independently of its origin).
Can you see me coming again to the top priority of maintaining the skin barrier to prevent allergies?
My opinion about it:
- Sounds quite good
- Need to wait for phase 3 trials
- Need to wait for long-term follow-ups, with more food challenges and blood tests to see if tolerance is maintained
How does it compare to the current two available oral peanut immunotherapy?
CA002 (Cambridge) and AR101 (London)
- Both use an “up-dosing” programme to achieve a specific tolerable dose (1600mg for the former and 600mg for the latter).
- AR101 had limitations, like participants being selected based on sensitivity to a maximum of 100 mg of peanut protein.
- This does not represent the entire population with peanut allergies, half of whom have reactions to doses >100 mg.
Michael R. Perkin, Consultant Paediatric Allergist PhD, wrote that the major concern regarding this immunotherapy is that 𝐚𝐥𝐥𝐞𝐫𝐠𝐞𝐧 𝐭𝐨𝐥𝐞𝐫𝐚𝐧𝐜𝐞 𝐭𝐡𝐚𝐭 𝐢𝐬 𝐢𝐧𝐝𝐮𝐜𝐞𝐝 𝐰𝐢𝐥𝐥 𝐛𝐞 𝐭𝐞𝐦𝐩𝐨𝐫𝐚𝐫𝐲, 𝐚𝐧𝐝 𝐥𝐨𝐬𝐭 𝐢𝐟 𝐫𝐞𝐠𝐮𝐥𝐚𝐫 𝐜𝐨𝐧𝐬𝐮𝐦𝐩𝐭𝐢𝐨𝐧 𝐜𝐞𝐚𝐬𝐞𝐬.
Neither group have attempted to establish the duration for which allergen tolerance is maintained in the absence of ongoing consumption, potentially lifelong, 𝐫𝐞𝐠𝐮𝐥𝐚𝐫 𝐜𝐨𝐧𝐬𝐮𝐦𝐩𝐭𝐢𝐨𝐧 𝐦𝐚𝐲 𝐛𝐞 𝐧𝐞𝐞𝐝𝐞𝐝 𝐭𝐨 𝐦𝐚𝐢𝐧𝐭𝐚𝐢𝐧 𝐚𝐥𝐥𝐞𝐫𝐠𝐞𝐧 𝐭𝐨𝐥𝐞𝐫𝐚𝐧𝐜𝐞.
𝐓𝐡𝐢𝐬 𝐦𝐞𝐚𝐧𝐬 𝐩𝐚𝐭𝐢𝐞𝐧𝐭𝐬 𝐰𝐨𝐮𝐥𝐝 𝐧𝐞𝐞𝐝 𝐭𝐨 𝐭𝐚𝐤𝐞 𝐭𝐡𝐞 𝐦𝐞𝐝𝐢𝐜𝐚𝐭𝐢𝐨𝐧 𝐨𝐧 𝐚 𝐝𝐚𝐢𝐥𝐲 𝐛𝐚𝐬𝐢𝐬 𝐨𝐫 𝐭𝐡𝐞𝐫𝐞 𝐰𝐢𝐥𝐥 𝐛𝐞 𝐫𝐞𝐯𝐞𝐫𝐬𝐚𝐥 𝐨𝐟 𝐬𝐲𝐦𝐩𝐭𝐨𝐦𝐬. 𝐀𝐧𝐝 𝐭𝐡𝐢𝐬 𝐰𝐚𝐬 𝐚𝐥𝐫𝐞𝐚𝐝𝐲 𝐩𝐫𝐨𝐯𝐞𝐧 𝐢𝐧 𝐚 𝐫𝐞𝐜𝐞𝐧𝐭 𝐬𝐭𝐮𝐝𝐲.
To consider that AR101 will cost between $5000 to $10,000 for the first six months of use, and $300 to $400 per month after that.